Complete the Mechanism for the Reaction of Pent-2-one
Reaction of cyclohex-2-en-1-one with CH3. Pent-3-en-2-one C5H8O CID 12248 - structure chemical names physical and chemical properties classification patents literature biological activities safety.

Pentan 2 One An Overview Sciencedirect Topics
231 Mechanism of Carbonyl Condensation Reactions An enolate of one carbonyl nucleophile reacts with the carbonyl carbon electrophile of a second carbonyl compound 12-addition reaction resulting in the formation of a new C-C bond General mechanism Fig.

. Complete the following mechanism involving 3-. In order to ensure that the oxidation to ethanoic acid is complete the reaction is carried out under reflux. Draw step 1 of the acid catalyzed addition of water to cis-3-methyl-2-pentene.
277 b Both faces are equally susceptible to attack so the alcohol product is racemic and most hydride reagents discussed. 15 points Write complete names for each of the following including stereochemistry if it is specifically shown. For primary carbocations that would have been formed the hydride shift occurs with the departure of the leaving group in one step to avoid forming such an unstable cation.
For higher-substituted carbocations this occurs more often in two steps. Future versions of this site may rely on reaction search pages in place of the enumerated reaction displays seen below. 15 points Complete each of the following reactions by adding the missing part.
I Use your knowledge of organic reaction mechanisms to complete the mechanism for this step by drawing two curly arrows on the following equation. Exam 2 Answer Key. Draw a reaction energy diagram for the addition of HBr to 1-pentene.
The mechanism if the reaction is given here. 2 6. 1 Answer to Complete the mechanism and the products for the reaction of 2-pentene with N-bromosuccinimide NBS in light and carbon tetrachloride.
The epoxidation of trans-pent-2-ene produces a racemic mixture of trans-pent-2-ene epoxide isomers. Basically according to the theory of hyperconjugation the addition of hydrogen bromide to 2-pentene would produce a predominane of 2-bromopentane since it is possible to write three hyperoonjugative structures involving the three -hydrogens of the methyl group but only two forms involving the two hydrogens of the ethyl group. Use curved arrows bonds atoms and electrons to complete the mechanism and products.
Give the reaction conditions which will complete the following transformations. I Name and outline a mechanism for the conversion of 2-bromopentane into pent-2-ene as shown below. Let one curve on the diagram show the.
The β -elimination. The lone pair of electrons in water serves this function. Up to 24 cash back a One of the steps in the mechanism for Reaction 1 involves the replacement of the functional group by bromine.
When pentan-2-one is reduced to pentan-2-ol for example the carbonyl is a prochiral center Section 145 since addition of hydride from either of the two faces a or b of the carbonyl unit in pentan-2-one will generate the two enantiomers of pentan-2-ol. Either the starting compound the necessary reagents and conditions or the final major product. A One of the steps in the mechanism for Reaction 1 involves the replacement of the functional group by bromine.
Use curved arrows show the major and minor intermediates label the nucleophiles and electrophiles and the order of the carbocations. The first two steps are completed. Use curved arrows bonds atoms and electrons to complete the mechanism and.
A general reaction search form is also available. Groups that were trans remain trans. Use a chair form and electron-pushing arrows to show the stereochemistry of the mechanism.
Show the structures of all intermediates and. Nucleophilic addition of HBr to 3-methylcyclohex-2-enone mechanism involving 3E-pent-3-en-2-one Carbonyl Compounds. Groups that were cis in the original alkene remain cis in the product.
CH3 NaNH 3 Reduction to trans alkene CH3 H2. Aldehydes ketones esters amides and nitrile. 10 points Write a complete mechanism for the E2 reaction of cis-1-bromo-2-methylcyclohexane with KOH in ethanol to form 1-methylcyclohexene.
The 12-hydride shift occurs to achieve a more stable carbocation intermediate. Compare the epoxidation of trans-but-2-ene. Individual Reactions.
Complete the mechanism and the products for the reaction of 2-pentene with N-bromosuccinimide NBS in light and carbon tetrachloride. Name and outline the mechanism of reaction 1 halogenoalkane for the formation of pent-1-ene. Up to 24 cash back The following conversions illustrate a number of different types of reaction mechanism.
Propose a mechanism to account for the following reactions. If we replace a methyl. Stereochemistry of Hydroboration-Oxidation.
The cracking of one molecule of compound X produces pent-1-ene ethene and butane in a 121 mol ratio. Step 1 In step 2 the carbocations electrophiles need nucleophiles to react with. Tell you about the mechanism of this reaction.
The addition is a concerted syn addition. A When 2-bromopentane reacts with ethanolic KOH two structurally isomeric alkenes are formed. IUse your knowledge of organic reaction mechanisms to complete the mechanism for this step by drawing two curly arrows on the following equation.
This page allows searching of all reactions involving this species. Write a half-reaction showing one reactant and its products Complete a material balance Use H 2O and H in acid solution Use H.

Pentan 2 One An Overview Sciencedirect Topics
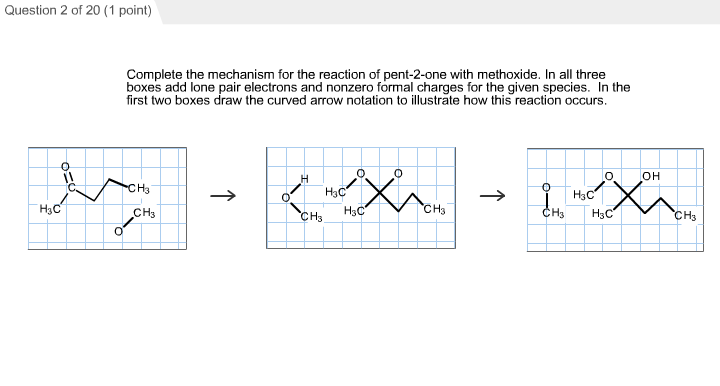
Solved Complete The Mechanism For The Reaction Of Pent 2 One Chegg Com
0 Response to "Complete the Mechanism for the Reaction of Pent-2-one"
Post a Comment